Is Clinical Trial Analytics Innovating as Fast as the Rest of Pharma?
The pharmaceutical industry is known for innovation and breakthroughs in medicine, but are we growing in all areas equally?
Boston Consulting Group reports that pharmaceutical companies make up a significant portion of self-reported ‘committed innovators’ – companies that say innovation is a top priority, and they support that commitment with significant investment. This is great news, as studies show that committed innovators are more likely to experience rising sales when compared to other segments. However, BCG reports that “even among committed innovators, only 60% report success in solving the challenges they prioritize.”
Innovation plays a key part in evolving our underlying systems and processes to drive success in clinical trial analysis. In this post we’ll look at 6 key areas of operation that play a role in clinical data analytics, and investigate problems being addressed and share some examples of solutions being implemented by modernization pioneers. For fun, we've given each area an innovation score as an indicator of how well we think that area is modernizing.
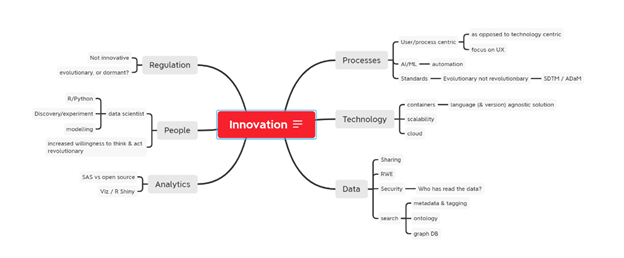
1. People
Teams grow and change as a result of many influences, including technology changes and cultural shifts. This past year, many organizations have been forced to embrace a willingness to think and act revolutionary. This includes the way we do work and how we collaborate, as remote working became the norm for most of 2020.
Another cultural shift making an impact on the industry is the transition to “data science”. Programmers are looking for new ways to work with data, including using different tools and methods. On top of this, new talent coming into organizations aren’t as familiar with legacy programs and are generally more comfortable using tools they’ve been trained on during formal education. This is placing pressure on organizations to evaluate technologies based on the way their people want to work.
What we’ve noticed:
- A transition to data science
- Employees are placing emphasis on upskilling, especially in non-traditional programming languages such as R and Python
- Teams are more open to discovery and experimentation
- Modelling
- New talent have updated expectations
- Organizations are showing an increased willingness to think and act revolutionary
- New ways of working and collaborating have been adopted more readily due to enforced remote working during the pandemic
|
Innovation Score: |
 |
2. Processes
Are your processes working for or against you? Many life science teams are looking to uncover hidden efficiencies in the way they work vs the tools they use to execute work. This places increasing focus on the user experience and using technology in a way that supports our processes instead of dictating them.
What we’ve noticed:
- Becoming user or process centric – adapting the organization and technology to optimized processes
- Automation to support processes, mitigate errors and increase efficiencies
- AI/ML
- Metadata
- A standards revolution – looking at standards in an evolutionary light vs revolutionary
- SDTM/ADaM
|
Innovation Score: |
 |
3. Technology
Technology is constantly evolving and we’re seeing some big changes that not only excite IT teams but their business counterparts as well.
Ten to fifteen years ago virtualization became a great way to help manage your data center and now we’re seeing the same sort of advancements when it comes to cloud computing and utilizing cloud providers (AWS, Azure, Google Cloud) to quickly spin up virtual servers that can change size as needed is allowing for greater scalability.
Fast forward, and we now have access to containers - which are so much more manageable than virtual machines. Containers offer pharmaceutical companies the ability to have multiple little servers that are set up for individual use cases (e.g. different software versions, programming languages, packages, validation requirements etc.). This functionality offers more flexibility and agility than we’ve had access to in the past.
These advancements are exciting to IT teams, and business teams alike once they realize that these technologies allow for reproducible outcomes and consistent results.
What we’ve noticed:
- Increased usage of the cloud
- Focus on the science, not the technology
- Focus on services, not servers (“server-less”)
- Increased scalability
- Cloud computing allows for elasticity when it comes to usage – pay only for what you use
- Get what you need when you need it
- Use of Containers (Docker and Kubernetes)
- Utilizing containers provides flexibility
- They can provide language and version agnostic solutions
- Upgrading is made easy
|
Innovation Score: |
 |
4. Data
Data is pharma’s number one asset. How do we make the most of it? How do we share and most importantly, protect it? We're seeing regulatory run-ins regarding data management more and more. Can you prove that rules are in place and show who has read or accessed the data at any given point in time?
What we’ve noticed:
- Sharing – the desire to share both internally and externally is rising
- Introduction of new data sources (real world data, wearables data)
- Security – maintaining access controls and audit trails
- Search – utilizing metadata and tagging, ontologies and graph databases to make understanding data easier
|
Innovation Score: |
 |
5. Analytics
While SAS has been the programming language of choice in the industry for many years, companies are now supplementing this with additional open source options such as R, Python, Julia, etc. We’re careful to advise our clients to consider how they’ll create a level playing field when it comes to using any combination of languages to ensure they’re making the best use of their compute resources.
Visualization tools are another gamechanger when it comes to analytics. Visualizing your data through graphs and charts can help identify patterns and errors, enabling your team to make data-driven decisions. We’ve seen an uptick in interest in programs like R Shiny and Tableau for this purpose.
Lastly, the sheer volume of data being collected and analyzed is growing. In order to have performant analytics, teams need to house their data close to their compute. Otherwise, you’re having to move a lot of data. Thinking about your data flow is extremely important as you’re architecting new environments.
What we’ve noticed:
- SAS vs Open Source
- Visualization tools are helping companies make sense of data through graphs and charts
- R Shiny is increasing in popularity, but how to deploy is proving a tricky obstacle
- Data volumes are increasing, so extra attention needs to be given to environment architecture
|
Innovation Score: |
 |
6. Regulation
Regulators are required to be a bit risk-averse and remain able to accept submissions from companies all over the world with varying levels of access to technology and advancement. So, what does this mean for innovation in regulation? Ultimately, we just need to prove consistency, that we can reproduce results, and show that processes are reliable. Does this require purchasing a new, expensive audit trail tool? Not necessarily.
We need to adhere to regulations, but they’re not currently driving innovation.
In the future, it would be interesting to see a change in how study reports are delivered to regulators and how regulators (and sponsors) understand the data. Moving away from PDFs including paper TFLs and getting closer to the data through a more interactive toolset, possibly with some visualization and a better way to share between sponsors and regulators
What we’ve noticed:
- Regulators haven’t made many advancements since the 1990s, however, this is understandable due to accessibility
|
Innovation Score: |
 |


